— Why choose SpectronRX / HymonBio?
— NeoVect Laboratory in the United States gave a feedback:
"Our primary concern was both logistics and science. A better and faster way to extract quickly and reliably the RNA with unique and novel chemistry and not held hostage to current supply chain issues. We have discovered through SpectronRx and HymonBio how to simplify the way nucleic acids are extracted in one easy step using their single-tube RNA extraction."
— Alex Ryder, MD, PhD, NeoVect Laboratories

No Centrifugation for sample separation and processing
No Beads, No Columns. Single tube 10 min
Save up to $10.00* per specimen with improved one step extraction
No need for a “hands on” front-end separation platform.
We save you time for prep, adding more tests per day.
Reduced human error with less hands-on time means less risk of contamination
Faster time to results for your practice and less anxiety for the participant/patient
* Dr Alex Ryder, NeoVect Labs
Hymon® SARS-CoV-2 Kit is a real-time RT-PCR for detecting COVID-19 in upper respiratory tract specimens (nasal, middle turbinate, nasopharyngeal or oropharyngeal swab specimens) and bronchoalveolar lavage specimens in patients of suspected cases.
FAST: Complete results in < 1.5 hours, from start to finish
HIGH CAPACITY: Test up to 96 samples in a single round of PCR analysis
SENSITIVITY: Detects 1.2 copies of SARS-CoV-2 RNA /uL, = 5 copies per reaction, with a positivity rate of >95%
NO INTERFERENCE: By the presence of interfering substances (substances commonly found in respiratory specimens, such as antibiotics, blood, mouthwash and sputum)
LOW COST: Complete set of reagents, buffers and controls are included, with no additional reagents required
PRACTICAL: The laboratory covers minimal area
UNIVERSAL: Perfect for remote or mobile testing
RELIABLE: Validated for use with the ABI-7500 and the QuantStudio5 PCR Systems
Clinical Evaluation showed 100% performance agreement with other commercially available coronavirus nucleic acid diagnostic kit.
Test targets the N and E gene sequences of SARS-COV-2 as amplification target regions.
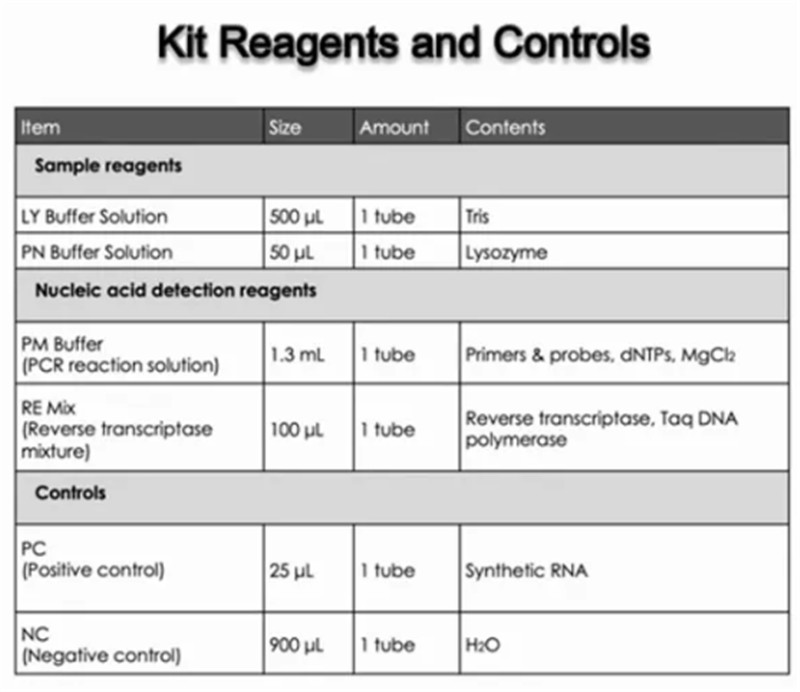
Positive Control for the Hymon® SARS-CoV-2 Test Kit consists of synthetic RNA of the SARS-CoV-2 N gene and E gene, as well as plasmid of human ACTB gene segment. The concentration of the positive control is 300 copies/mL.

One-Step Simple Process

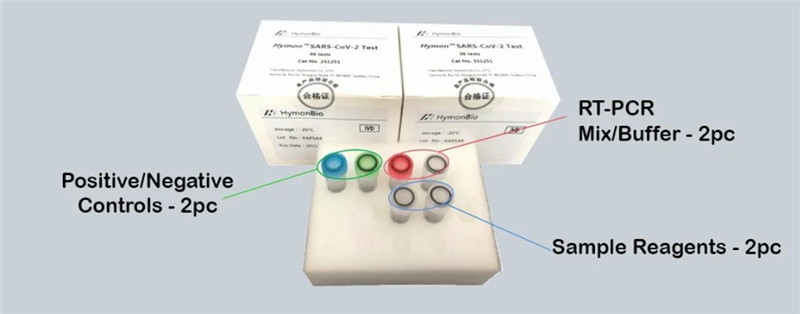
Hymon® SARS CoV-2 Kit—Simple Test Kit
Post time: Sep-07-2020

