The 8th “Voice of Creation” China Experimental Medicine Conference (CEMC) and the 20th China International Laboratory Medicine and Blood Transfusion Instrument and Reagent Expo (CACLP) will be held in Nanchang Greenland International Expo Center from May 28th to May 30th. HymonBio will make a brand new debut with innovative products such as: tumor molecular detection, pathogen molecular detection, nucleic acid sample processing, etc.
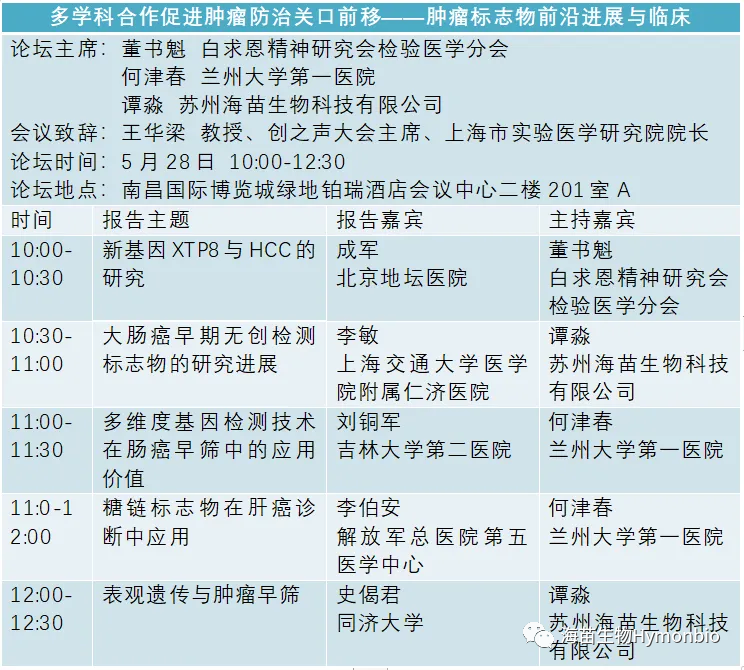
The Creative Voice Special Forum, exclusively sponsored and co organized by HymonBio, will reveal the latest progress in early cancer screening. It aims to promote international and interdisciplinary cooperation in advancing the threshold of cancer prevention and treatment, bringing the latest progress in clinical research on tumor markers and an academic feast of innovative interdisciplinary cutting-edge technologies. The forum is chaired by Professor Dong Shukui from the Laboratory Medicine Branch of the Bethune Spiritual Research Association, Chairman of the Experimental Medicine Technology Transformation Special Committee; Professor He Jinchun from Lanzhou University First Hospital; and Dr. Tan Miao, a member of the Experimental Medicine Technology Transformation Special Committee and Chairman of HymonBio Co., Ltd. Professor Wang Hualiang, Chairman of the Creative Voice Conference and Dean of Shanghai Experimental Medicine Research Institute, delivered a speech at the conference.
Established in 2015, HymonBio Co., Ltd. is a high-tech enterprise focusing on the research and development, production and sales of innovative molecular diagnosis and POCT related products. The company has 11 products that obtained China medical device certificate, 59 products with EU CE certificate, 1 product with FDA EUA certificate, applied for 36 patents, and obtained 16 authorized patents.
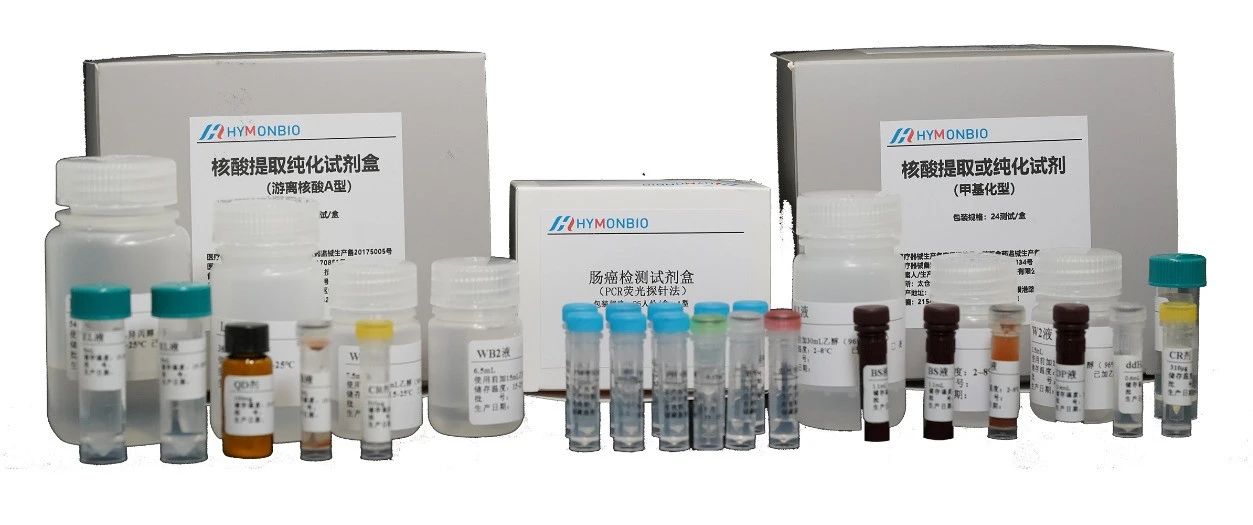
HymonBio’s R&D team has rich experience in developing molecular testing and has served as project leader for sub-projections of national major special testing products. The company has successively won honors of High-Tech Enterprise, Jiangsu R&D Enterprise, Jiangsu Province Private Science and Technology Enterprise, Jiangsu Province “Double Innovation Plan” Supporting Enterprise, Gusu Innovation and Entrepreneurship Leading Talent Enterprise, Taicang Innovation and Entrepreneurship Leading Talent Enterprise, Taicang City Pioneer Enterprise, etc. The Colorectal Cancer Early Detection Screening Test developed by HymonBio covers three gene methylation and one gene mutation; it adopts unique ECS Magnetic Bead Extraction Technology and MoLock Molecular Lock Amplification to improve extraction efficiency of low-concentration small fragment cell-free nucleic acid. Clinical studies have shown the kit has a sensitivity of 96% and specificity of 93%
Above: The Colorectal Cancer Screening Panel: Nucleic Extraction Kit, Methylation Conversion Kit, and Colorectal Cancer Screening Test
The company is committed to the development of a series of early cancer screening technologies and products in addition to colorectal cancer, including high-sensitivity/high-specificity gastric cancer and bladder cancer screening kits.
Left: Gastric Cancer Screening Kit
Right: Bladder Cancer Screening Kit
During the SARS-CoV-2 epidemic, HymonBio developed the innovative “one-step” method that allows automatic release of the viral nucleic acid in 3 minutes, with sample-to-results in 30 minutes. Combined with the DIDC Dual Internal Standard Design, sensitivity reaches 200 copies/mL and self-monitored, effectively avoiding false positives and false negatives. It has successfully obtained FDA EUA, CE certification, and other national qualification certificates. It has been reported by more than 30 overseas media, and mentioned by Dr. Stephen M. Hahn, former commissioner of the US FDA. To date, it has received no complaints worldwide.
In addition, HymonBio has developed freeze-dry technology, which relieves the need for cold chain storage and significantly reduces shipping costs, making it suitable for long-distance transportation. This technology has been applied to a full range of other molecular detection products, including the Multiplex Respiratory Panel Test Kit and Urogenital Pathogens Panel Test Kit, which have been exported to multiple countries.
Introduction to the Agenda of the Creative Voice Special Forum
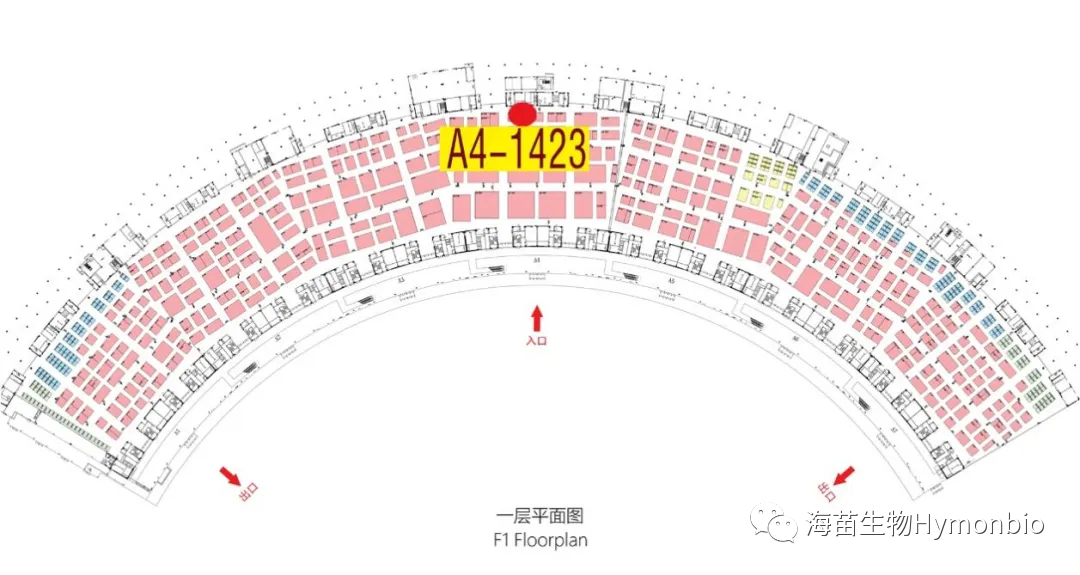
Special forum meeting location: Room 201, 2nd floor, Conference Center, Greenland Platinum Hotel
CACLP Exhibition HymonBio Booth: A4-1423
All who visited our booth on-site has the opportunity to receive a “Chinese-French-English Medical Term Dictionary” and gifts from HymonBio.
Post time: May-11-2023