-

Fast delivery Rna Virus Test - Colosafe™ Colon Cancer Gene Detection (Fluorescence PCR) – HymonBio
Product Description Colosafe™ coloretectal cancer DNA screening test (fluorescence PCR method) has passed CE certification and is used for early colorectal cancer screening. -

China Factory for Sansure Test Reagent Kit – Nucleic Acid Extraction and Purification Kit (Solid Phase Matrix Method) – HymonBio
Product Description Specification: 1 test / box The reagent can be used to purify nucleic acids in whole blood, swab samples and other body fluid samples. This product mainly provides purified high-quality nucleic acid for downstream nucleic acid amplification experiments or other enzyme kinetics experiments. -
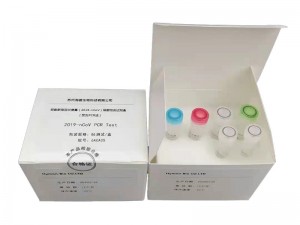
2021 New Style Quick Test Pcr - Hymon® SARS-CoV-2 Test Kit – HymonBio
Hymon® SARS-CoV-2 Test Kit COVID-19 RNA Test Kit By HymonBio Co., Ltd. Hymon® SARS-CoV-2 Test Kit provides in vitro detection of coronavirus SARS-CoV-2 in nasopharyngeal swab and other respiratory samples. The virus infects human and caused COVID-19. ● 40-90 mins to results ● 94-sample capacity in one kit (+ controls) ● LOD: 1.2 copies/uL = 5 copies/reaction ● All reagents included ● Small lab footprint ● Perfect for remote or mobile testing ● Low cost Video FAST ONE-STEP PR... -

Top Suppliers Rapid And Rt Pcr - Colosafe™ Colon Cancer Gene Detection (Fluorescence PCR) – HymonBio
Product Description Colosafe™ coloretectal cancer DNA screening test (fluorescence PCR method) has passed CE certification and is used for early colorectal cancer screening. -

Super Purchasing for Quick Result Pcr Test - Colosafe™ Colon Cancer Gene Detection (Fluorescence PCR) – HymonBio
Product Description Colosafe™ coloretectal cancer DNA screening test (fluorescence PCR method) has passed CE certification and is used for early colorectal cancer screening. -

High reputation Nucleic Acid Amplification Test Rapid Test - Colosafe™ Colon Cancer Gene Detection (Fluorescence PCR) – HymonBio
Product Description Colosafe™ coloretectal cancer DNA screening test (fluorescence PCR method) has passed CE certification and is used for early colorectal cancer screening. -

Good Quality Virus Sampling Tube - Hymon® SARS-CoV-2 Test Kit – HymonBio
Hymon® SARS-CoV-2 Test Kit COVID-19 RNA Test Kit By HymonBio Co., Ltd. Hymon® SARS-CoV-2 Test Kit provides in vitro detection of coronavirus SARS-CoV-2 in nasopharyngeal swab and other respiratory samples. The virus infects human and caused COVID-19. ● 40-90 mins to results ● 94-sample capacity in one kit (+ controls) ● LOD: 1.2 copies/uL = 5 copies/reaction ● All reagents included ● Small lab footprint ● Perfect for remote or mobile testing ● Low cost Video FAST ONE-STEP PR... -

Massive Selection for Rapid Pcr Swab - Colosafe™ Colon Cancer Gene Detection (Fluorescence PCR) – HymonBio
Product Description Colosafe™ coloretectal cancer DNA screening test (fluorescence PCR method) has passed CE certification and is used for early colorectal cancer screening. -

China Supplier Omicron Rapid Test Kit - Colosafe™ Colon Cancer Gene Detection (Fluorescence PCR) – HymonBio
Product Description Colosafe™ coloretectal cancer DNA screening test (fluorescence PCR method) has passed CE certification and is used for early colorectal cancer screening. -

8 Year Exporter Viral Rna/Dna Sample Hold - Colosafe™ Colon Cancer Gene Detection (Fluorescence PCR) – HymonBio
Product Description Colosafe™ coloretectal cancer DNA screening test (fluorescence PCR method) has passed CE certification and is used for early colorectal cancer screening. -

Manufacturer for Freeze-Dried Pcr Kit - Colosafe™ Colon Cancer Gene Detection (Fluorescence PCR) – HymonBio
Product Description Colosafe™ coloretectal cancer DNA screening test (fluorescence PCR method) has passed CE certification and is used for early colorectal cancer screening. -

2021 China New Design Nucleic Acid Based Assays - Hymon® SARS-CoV-2 Test Kit – HymonBio
Hymon® SARS-CoV-2 Test Kit COVID-19 RNA Test Kit By HymonBio Co., Ltd. Hymon® SARS-CoV-2 Test Kit provides in vitro detection of coronavirus SARS-CoV-2 in nasopharyngeal swab and other respiratory samples. The virus infects human and caused COVID-19. ● 40-90 mins to results ● 94-sample capacity in one kit (+ controls) ● LOD: 1.2 copies/uL = 5 copies/reaction ● All reagents included ● Small lab footprint ● Perfect for remote or mobile testing ● Low cost Video FAST ONE-STEP PR...

